Incubator Research
Learn how our innovative early-stage research projects are laying the groundwork for future treatments.
Incubator Research
Innovation lies at the heart of the Bionics Institute and early-stage ideation is strongly supported through the Bionics Incubator Fund (BIF).
To apply for funding, researchers pitch an idea for a new medical device or adaptation of existing technology for a different condition.
Ideas funded by the BIF have led to several successful projects, including:
Abdominal vagus nerve stimulation as a novel treatment of rheumatoid arthritis, which is detailed here, was initiated by Sophie Payne, James Fallon and Amy Morley.


Improving residual hearing function in cochlear implant patients with gene therapy
Researchers at the Bionics Institute are exploring the use of gene therapy to regenerate lost hair cells in the inner ear, that mediate sound and balance. A large, and growing subset of cochlear implant recipients have some residual hearing in the low frequencies, which enables them to better perceive fine pitch differences that are important for speech recognition in noisy environments and improved appreciation of music. However, a significant number of cochlear implant recipients experience a progressive loss of hearing over time. This research will significantly improve the outcomes of patients with cochlear implants.
Bionics Institute researchers: Dr Niliksha Gunewardene, Professor James Fallon, A/Prof Andrew Wise and A/Prof Rachael Richardson.
Optogenetic hybrid stimulation of peripheral nerves
Researchers at the Bionics Institute are investigating the potential of optical stimulation to improve precision in the electrical stimulation delivered by medical devices. The peripheral nervous system has accessible connections with the body’s organs and systems providing the opportunity to treat an increasing number of conditions with electric medicine techniques. However, a limitation of delivering electrical stimulation in mixed peripheral nerves is the difficulty in discriminating between functional fibre groups, leading to unwanted side effects and reduced treatment efficacy. This research promises to expand the range of health conditions that can be treated with bionic devices.
Bionics Institute researchers: A/Prof Rachael Richardson, Dr Sophie Payne, Professor James Fallon, Dr Niliksha Gunewardene, Dr Alex Thompson, Dr Mary Ardren and Elise Ajay.

Counting the cost – three-dimensional quantification of the cochlea
Researchers at the Bionics Institute are working to develop a method of analysing cellular morphology within an intact cochlea to provide a high resolution, three-dimensional image where cells can be qualitatively and quantitatively assessed. The complexity of the cochlea, a structure in the inner ear, presents many challenges to researchers wishing to study hearing. Traditional histological preparation of the cochlea via sectioning and staining is not only time and labour intensive but can only provide a partial understanding of the cellular morphology of the tissue. If realised, this technique could enable unprecedented insight into the response of the cochlea to a wide variety of injuries and treatments.
Bionics Institute researchers: Ella Trang, Alex Hill, Lisa Dyball and Patrick Lam.

Developing an objective measure of tinnitus in a pre-clinical model
The Bionics Institute has developed an objective test of tinnitus in humans, using a non-invasive brain imaging technique called functional near-infrared spectroscopy (fNIRS) together with machine learning analysis techniques. An important application of this objective test is evaluation of therapeutic efficacy of tinnitus drugs. We now aim to adapt this measure for use in a pre-clinical animal model of tinnitus, to enable evaluation of the safety and efficacy of early-phase drugs. Adapting our fNIRS-based objective measure of tinnitus to a pre-clinical model will create a unique platform at the Bionics Institute to objectively evaluate the effectiveness of tinnitus therapeutics and behavioural treatments, from early animal studies to human trials and clinical use.
Bionics Institute researchers: Mehrnaz Shoushtarian, James Fallon, Michelle Bravo.

Discovering new disease targets for vagus nerve stimulation
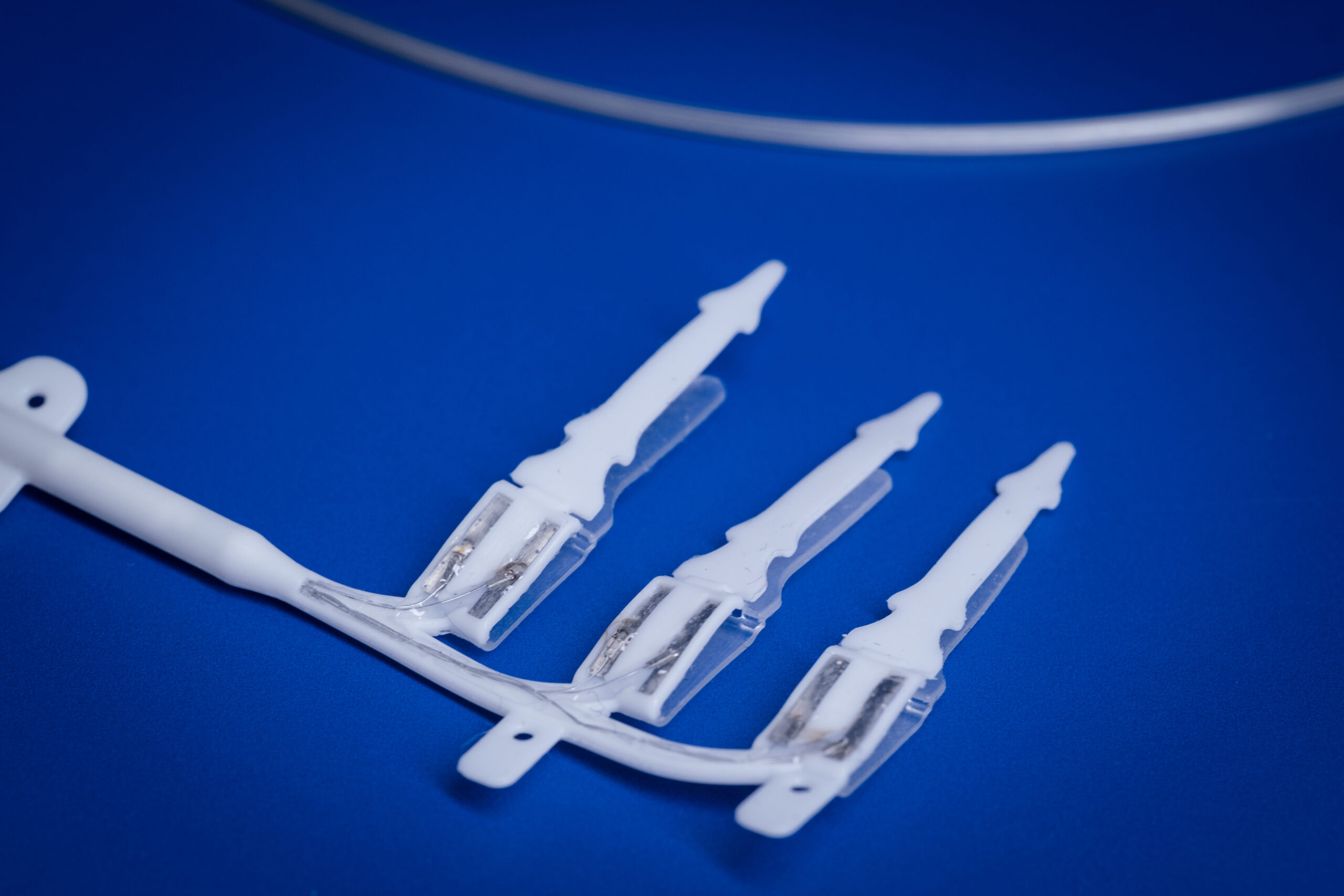
This project will expand the Bionics Institute’s vagus nerve stimulation technology and utilise novel thin-film fabrication techniques to develop a miniaturised peripheral nerve array for the abdominal vagus nerve. We have developed a cuff electrode array designed for long-term implantation onto the abdominal vagus nerve. This technology has been used to validate the safety and efficacy of vagus nerve stimulation to treat Crohn’s disease and rheumatoid arthritis. There are numerous mouse genetic models of human diseases, which are an essential tool for understanding mechanisms of disease and discovering new therapies and the aim of this There are numerous mouse genetic models of human diseases, which are an essential tool for understanding mechanisms of disease and discovering new therapies and the aim of this project is to assess which other diseases could be treated by our abdominal vagus nerve technology.
Bionics Institute researchers: Professor James Fallon and Dr Sophie Payne
Investigating the effect of abdominal vagus nerve stimulation on the brain
The aim of this project is to expand the application of abdominal vagus nerve stimulation (VNS) for treatment of brain disorders. We are particularly interested in the area of the brain responsible for the therapeutic effects of cervical VNS for treatment of epilepsy and depression. The advantage of abdominal VNS developed at the Bionics Institute is that it is free from respiratory and cardiac side effects associated with conventional cervical VNS in the neck. If abdominal VNS can activate the therapeutic brain region similarly to cervical VNS, it has a potential to be used as a more effective alternative to cervical VNS for a range of brain disorders.
Bionics Institute researchers: Tomoko Hyakumura, Sophie Payne, Jerico Matarazzo, Wendy Adams and James Fallon
Developing miniaturised peripheral nerve interface technology for the vagus nerve
Using electricity to alter the activity of nerves, dubbed ‘electroceutical therapy’, is a viable alternative to drug treatment. In particular, stimulation of the vagus nerve is an effective treatment of a wide range of diseases including inflammatory bowel disease, rheumatoid arthritis, heart failure, obesity and gastroparesis. The overall goal of this proposal is to develop a miniature device for long-term use in the mouse utilising innovative fabrication techniques. This project will focus on the design, fabrication, testing and validation of a new device intended for implantation onto the mouse abdominal vagus nerve.
Bionics Institute researchers: Dr Sophie Payne, Professor James Fallon and Dr Tomoko Hyakumura
Eavesdropping in the sciatic nerve: validating new recording technology
A fundamental limitation with existing neural recording technology in the peripheral nervous system is the trade-off between selectivity (i.e. ability to record from different fibre types) and invasiveness (i.e. damage sustained to the nerve). Here we aim to demonstrate a new recording and signal processing technology that is highly selective and allows the type (sensory/motor) and class (fast/slow) of neural activity to be identified with minimal damage to the nerve. This technology will have significant implications as a neural mapping research tool to acquire deeper understanding of peripheral circuits, and profound impact on the development of adaptive neural prosthesis systems.
Bionics Institute researchers: Dr Sophie Payne, Professor James Fallon, Dr Tomoko Hyakumura and Jerico Matarazzo.
Refining deep brain stimulation with optogenetics
Deep brain stimulation (DBS) is well-established treatment for Parkinson’s disease. Although beneficial, limited precision of neural activation and serious side-effects remain major issues with this therapy. We recently discovered that hybrid stimulation, a combination of electric and optogenetic stimulation, improves the precision of neural activation. This research will explore the potential of this technology to improve the selectivity of neural stimulation via DBS.
Dr Niliksha Gunewardene, Assoc. Professor Rachael Richardson, Prof James Fallon, Dr Tomoko Hyakumura, Kyle May and Jerico Matarazzo

Magnetic Percutaneous Plug
This project was initiated to benefit both the animals we work with and the researchers who care for them. All previous designs of rodent percutaneous connectors (the interface between an implanted array and external stimulating/recording device) have used regular pin termination connectors that require a degree of force to connect. This project has developed a magnetic connector for animal research that removes any need for force when connecting. Such a simple modification has allowed for a marked improvement to animal welfare as it has made connection and disconnection of the percutaneous connector a seamless and simple exercise for researchers at the institute
Bionics Institute researchers: Jerico Matarazzo, Dr Sophie Payne, Dr David Hill, Ross Thomas
Optimising Haematoxylin and Eosin Staining for Resin Embedded Tissue Sections
This completed project aimed to develop a haematoxylin and eosin (H&E) staining technique for use in resin embedded tissue samples. The scientific field of neuromodulation activates neural tissue using electrode arrays that are currently being developed by the Bionics Institute. It is important to assess how the neural tissue contacts the electrode interface in situ to further inform and develop the field of neural recordings and peripheral nerve interface design. Currently, we use a metachromatic toluidine blue stain on our resin samples, which does not differentially stain the tissue. H&E staining allows for the distinction between different tissue types, enabling more precise histological analysis on how the neural tissue interacts with the electrodes. This project was completed and was considered a success. This is an example of a successful incubator project that has helped our research capabilities at the Bionics Institute.
Bionics Institute researchers: Chiara Braida, Dr Sophie Payne, Ella Trang, Lisa Dyball