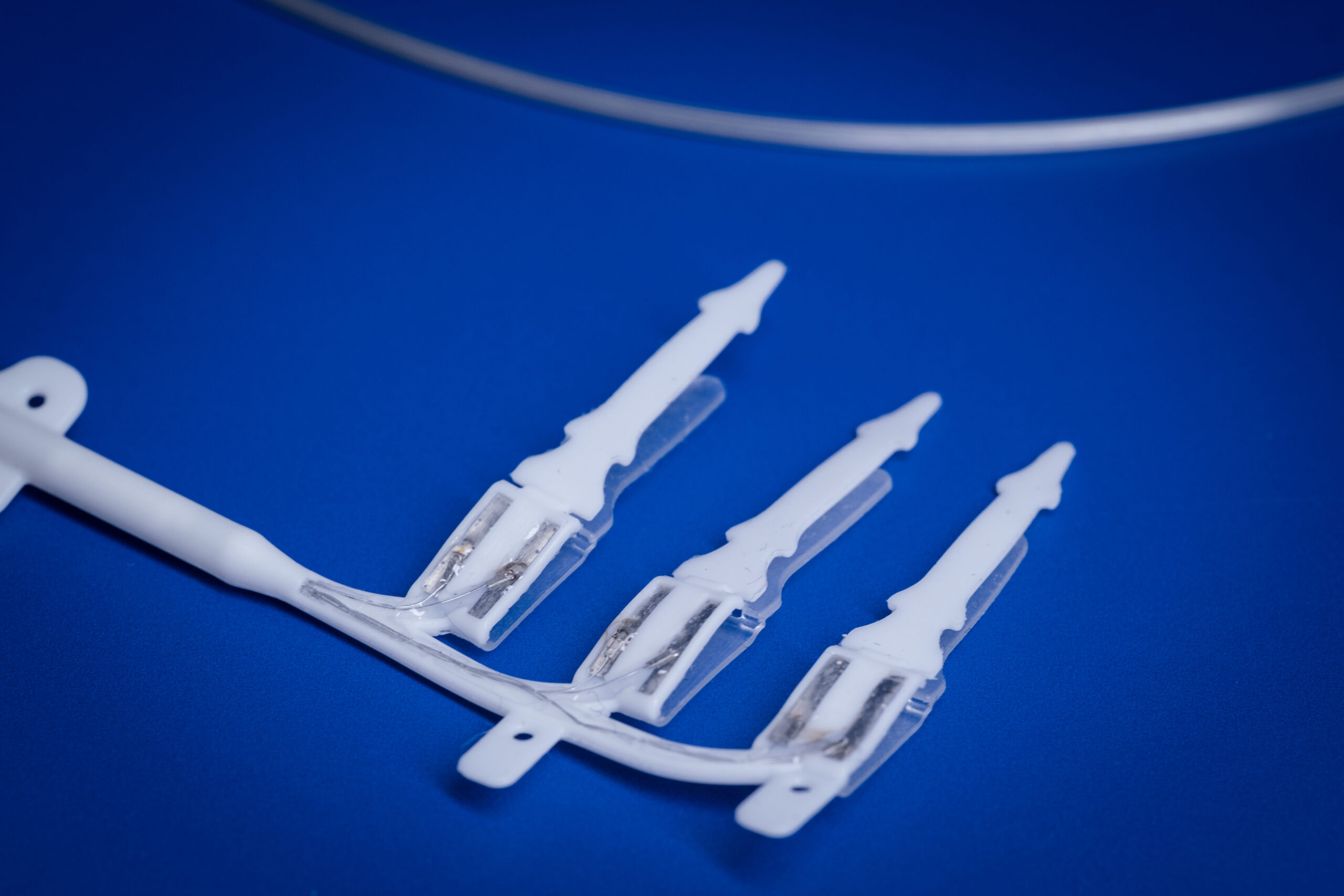
A/Prof Rachael Richardson, Associate Professor
A/Prof Rachael Richardson is a Principal Research Fellow at the Bionics Institute, and Associate Professor in the Medical Bionics Department, University of Melbourne.
She has a background in molecular biology (Walter and Eliza Hall Institute) and hearing science (Bionics Institute).
The main goal of Rachael’s research is to develop innovative strategies to improve the spatio-temporal precision of neural modulation.
A fundamental limitation of cochlear implants, for example, is the spread of electrical current which means that nerves in one part of the cochlea can be activated by more than one electrode.
Rachael is working on using light to activate the hearing nerve as light can be focused for more precise activation.
The main goal is to determine whether light stimulation alone or in combination with electrical stimulation can significantly improve the precision of nerve activation in the cochlea and whether this will result in a meaningful difference to the way people hear sound.
Rachael is also applying this technology to the retina and the peripheral nervous system.
Another major research focus is protecting and restoring hearing.
Rachael is employing gene therapy and nano-technology-inspired drug delivery techniques to introduce therapeutics into the cochlea for hair cell regeneration or nerve survival and repair.
These techniques have the potential to improve the outcomes achieved with cochlear implantation or even restore hearing by repopulating the damaged sensory region of the cochlea with new cells.
Rachael is interested in drug delivery techniques, pharmacokinetics and functional assessment in order to help drug discoveries for hearing protection and restoration move towards clinical translation.
A/Prof Richardson has authored 50 peer-reviewed scientific papers, 3 book chapters and numerous conference presentations as invited speaker.
She has been the principal investigator on 11 major Australian and international project grants including NHMRC, and co-investigator on a further 4 major project grants, including the US Department of Defense, totalling over 5.2 million dollars.
She also works closely with industry on contract research projects. Rachael collaborates with research leaders and clinicians from the University of Melbourne, Swinburne University, RMIT and the University of Freiburg.
Rachael has been the primary or co-supervisor of 10 PhD/ Masters students, 5 honors students, 5 undergraduate research opportunity program students/interns and 2 advanced medical science students.
Many of these students have won multiple awards and recognition for their research. Rachael is currently accepting new students.
Research projects
- Optogenetics for precise neural modulation
- Protecting and regenerating sensory cells after hearing loss
- Gene therapy in the cochlea
- Protection of residual hearing during cochlear implantation
- Improving the nerve-electrode interface of the cochlear implant
- Optogenetics for optical stimulation of auditory neurons
Selected Publications:
1. RT Richardson, AC Thompson, AK Wise, EA Ajay, N Gunewardene, SJ O’Leary, PR Stoddart, and JB Fallon (2021) Viral-mediated transduction of auditory neurons with opsins for optical and hybrid activation Scientific Reports 11; 11229 https://doi.org/10.1038/s41598-021-90764-9
2. AC Thompson, AK Wise, W Hart, K Needham, JB Fallon, P Stoddart, N Gunewardene, RT Richardson (2020) Hybrid optogenetic and electrical stimulation for greater spatial resolution and temporal fidelity of cochlear activation Journal Neural Engineering, 17(5), 056046. https://dx.doi.org/10.1088/1741-2552/abbff0
3. Preprint: bioRxiv 2020.07.27.187294; doi: https://doi.org/10.1101/2020.07.27.187294
4. P Lam, N Gunewardene, Y Ma, F Caruso, T Nguyen, B Flynn, AK Wise and RT Richardson (2020) A radiolabeled drug tracing method to study neurotrophin-3 retention and distribution in the cochlea after nano-based local delivery MethodsX 7, 101078
5. WL Hart, RT Richardson, T Kameneva, AC Thompson, AK Wise, JB Fallon, PR Stoddart and K Needham (2020) Combined optogenetic and electrical stimulation of auditory neurons increases effective stimulation frequency – an in vitro study, J Neural Eng 17(1); 016069.
6. Y Ma, C Cortez-Jugo, J Li, X Lin, RT Richardson, Y Han, J Zhou, M Björnmalm, O Feeney, Q Zhong, C Porter, A Wise and F Caruso (2019) Engineering Biocoatings to Prolong Drug Release from Supraparticles, Biomacromolecules 20(9); 3425-3434
7. Y Ma, AK Wise, RK Shepherd, RT Richardson (2019) New molecular therapies for the treatment of hearing loss Pharmacology and Therapeutics 200; 190-209
8. RT Richardson, Q Hu, F Shi, T Nguyen, JB Fallon, BO Flynn and AK Wise (2019) Pharmacokinetics and tissue distribution of neurotrophin 3 after intracochlear delivery, February 15th 2019 Journal of Controlled Release 299; 53-63 DOI: 10.1016/j.jconrel.2019.02.018
9. Cover story and image: K Park (2019) Pharmacokinetic studies for cochlear drug delivery Journal of Controlled Release 299; 165 DOI:10.1016/j.jconrel.2019.03.007
10. Y Ma, M Björnmalm, A Wise, C Cortez-Jugo, E Revalor; Y Ju, O Feeney, R Richardson, E Hanssen, RK Shepherd, C Porter and F Caruso (2018) Gel-Mediated Electrospray Assembly of Silica Supraparticles for Sustained Drug Delivery. ACS Applied Materials & Interfaces 10(37):31019-31031 DOI:10.1021/acsami.8b10415
11. Richardson, R. T., A. C. Thompson, A. K. Wise, and K. Needham. 2017. Challenges for the application of optical stimulation in the cochlea for the study and treatment of hearing loss. Expert Opinion on Biological Therapy. 17(2): 213-23.
12. Wise, A. K., B. O. Flynn, P. J. Atkinson, J. B. Fallon, M. Nicholson, and R. Richardson. 2015. Regeneration of cochlear hair cells with Atoh1 gene therapy after noise-induced hearing loss Journal of Regenerative Medicine. 4(1): doi: http://dx.doi.org/10.4172/2325-9620.1000121. Full Text
13. Richardson, R. T., and P. J. Atkinson. 2015. Atoh1 gene therapy in the cochlea for hair cell regeneration. Expert Opinion on Biological Therapy. 15(3): 417-30.
14. Newbold, C., S. Mergen, R. Richardson, P. Seligman, R. Millard, R. Cowan, and R. Shepherd. 2014. Impedance changes in chronically implanted and stimulated cochlear implant electrodes. Cochlear Implants International. 15(4): 191-9.
15. Irving, S., L. Gillespie, R. Richardson, D. Rowe, J. B. Fallon, and A. K. Wise. 2014. Electroacoustic stimulation: now and into the future. Biomed Research International. 2014: 350504. doi: 10.1155/2014/04. Full Text
16. Gillespie, L. N., R. T. Richardson, B. A. Nayagam, and A. K. Wise. 2014. Treating hearing disorders with cell and gene therapy. Journal of Neural Engineering. 11(6): 065001. Full Text
17. Atkinson, P. J., A. K. Wise, B. O. Flynn, B. A. Nayagam, and R. T. Richardson. 2014. Viability of long-term gene therapy in the cochlea. Scientific Reports. 4: 4733. doi: 10.1038/srep04733. Full Text
18. Atkinson, P. J., A. K. Wise, B. O. Flynn, B. A. Nayagam, and R. T. Richardson. 2014. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS ONE. 9(7): e102077. doi: 10.1371/journal.pone.0102077. Full Text
19. O’Leary, S. J., P. Monksfield, G. Kel, T. Connolly, M. A. Souter, A. Chang, P. Marovic, J. S. O’Leary, R. Richardson, and H. Eastwood. 2013. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hearing Research. 298: 27-35. doi: 10.1016/j.heares.2013.01.012. Full Text
20. O’Leary, S. J., P. Monksfield, G. Kel, T. Connolly, M. A. Souter, A. Chang, P. Marovic, J. S. O’Leary, R. Richardson, and H. Eastwood. 2013. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hearing Research. 298: 27-35. doi: 10.1016/j.heares.2013.01.012. Full Text
(See more publications by Rachael Richardson in PubMed.
(See more publications by Rachael Richardson in Google Scholar.