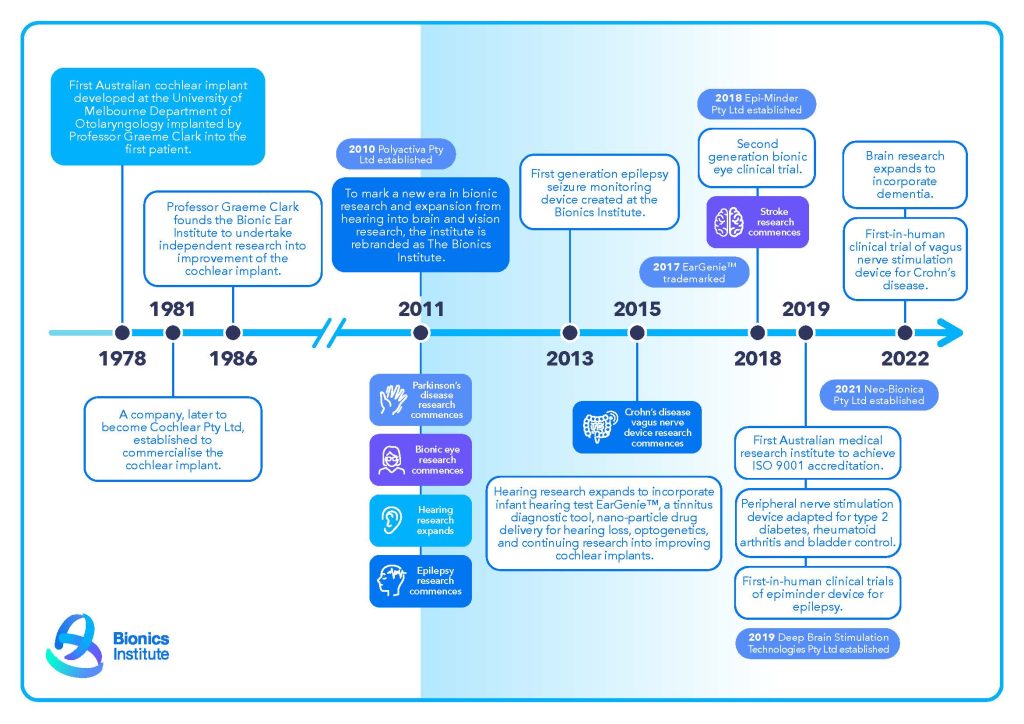
The Bionics Institute has an impressive track record of translating medical device concepts into clinical reality, dating back to 1986 when it was founded by Professor Graeme Clark.
Professor Clark led the team that created Australiaʼs cochlear implant, which has given hearing to more than a million deaf people around the world. To read more about Professor Clark’s achievements, click here.
Since then, Bionics Institute researchers have developed medical devices that could change the lives of people living with Alzheimer’s disease, hearing impairment, Parkinson’s disease, Crohn’s disease, epilepsy and rheumatoid arthritis.
This work has been overseen by leading researchers in Director and Chief Technology Officer roles, including Professor Rob Shepherd AM and Professor James Fallon. This includes:
Epilepsy seizure monitoring device, commercialised by Bionics Institute spin-off company Epiminder.
Infant hearing diagnostic tool commercialised by EarGenie
Innovative vagus nerve stimulation device to treat inflammatory and neurological conditions.
Novel drug delivery methods to restore hearing loss
Objective measure of tinnitus used to validate new treatments and provide diagnosis
Australiaʼs first-generation bionic eye prototype implanted in clinical trials in 2018, commercialised by Bionic Vision Technologies.
QMS ISO 9001

In 2019, the Bionics Institute became the first and only Australian Medical Research Institute to receive an internationally recognised accreditation for our Quality Management System (ISO 9001) covering all operations, research and development processes. This accreditation reinforces our commitment to generating real clinical impact from our research and differentiates us from other MRIs and Universities.
Neo-Bionica

In 2021 we launched the spin-off company Neo-Bionica, a joint initiative of the Bionics Institute and the University of Melbourne, which combines the engineering expertise and state-of-the-art facilities needed to develop medical devices from initial concept to first-in-human prototypes for clinical trials.