Latest News
Research Update July 2025
We couldn’t do what we do without your generous support. Here’s a roundup of the progress we have made with your help.
Alzheimer’s disease
“Nearly 30 people have been enrolled into our clinical trial investigating the use of brain stimulation as a potential treatment for Alzheimer’s disease out of a total of 132, and we are continuing to develop the technology with the aim of delivering highly personalised therapy.”
– Professor Kate Hoy
Balance disorders
“My team in the NeuroMovement Laboratory is working closely with Bionics Institute engineers to develop wearable sensors that collect real-time data on movement and coordination, allowing for a more accurate assessment of a patient’s balance condition. It has the potential to detect early signs heightened falls risk, even before they become noticeable to the patient or healthcare provider.”
– Associate Professor David Szmulewicz
Chronic pain
“The Bionics Institute has been researching the use of a combination of light and electricity to improve cochlear implants. We recently started applying this technique to treat chronic pain, and early results are looking promising.”
– Professor Rachael Richardson
Crohn’s disease
“The first patient in the clinical trial of our vagus nerve device to prevent inflammation in Crohn’s disease has completed his 18 months in the trial and continues to feel fit and well. With the support of the Helmsley Charitable Trust we are continuing research into an innovative way to refine the device to provide adaptive stimulation.”
– Professor James Fallon, Chief Technology Officer
Rheumatoid arthritis
“In June this year, we launched our clinical trial to assess safety of vagus nerve stimulation and its potential benefits in reducing swelling and pain caused by rheumatoid arthritis. The Bionics Institute is the sponsor, and the trial is run in collaboration with our clinical team from St Vincent’s Hospital and the Austin Hospital.”
– Associate Professor Sophie Payne
Tinnitus
“We are collaborating with several clinicians to use our tinnitus diagnosis technology to monitor potential tinnitus treatments under investigation, with the aim of finding new ways to relieve symptoms.”
– Associate Professor Mehrnaz Shoushtarian
Infant hearing
“We are engaging with leading clinicians worldwide to refine our EarGenie test of infant hearing so that, once approved for clinical use, it will fast track intervention for infants with hearing loss to optimise their language development. I am excited to be one of the Founders of a newly created company, EarGenie Pty Ltd, which is currently completing its early fundraising activities, to be the vehicle to undertake ongoing engineering and clinical development and to ultimately market products to the audiology community throughout the world.”
– Professor Colette McKay
Hearing loss
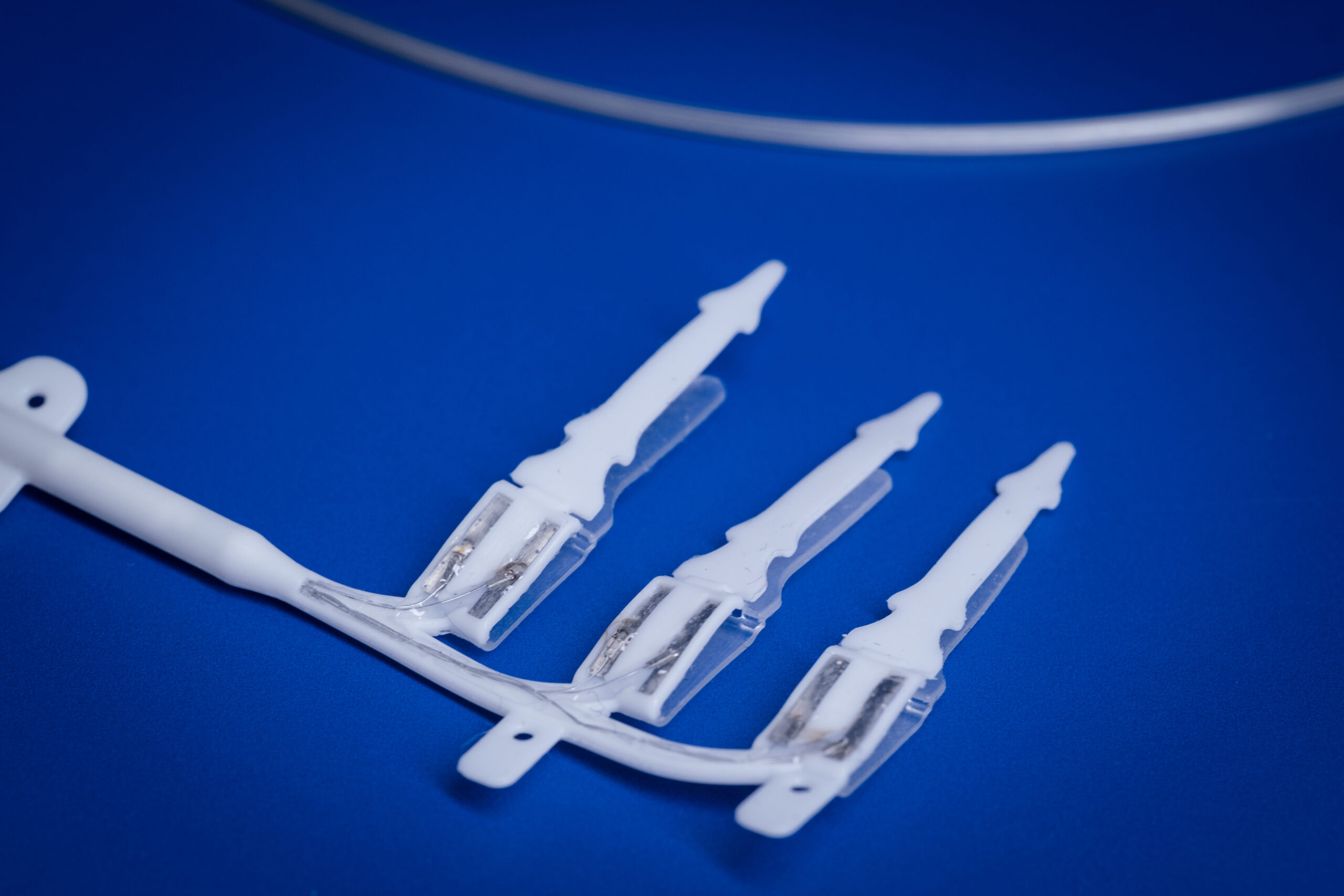
“We are currently developing the process to manufacture a clinical grade drug delivery system for potential use in a clinical trial of our innovative therapy that aims to improve quality of life for people with hearing impairment.”
– Associate Professor Andrew Wise
Epilepsy
“We recently published a scientific journal paper on our early research into the potential use of vagus nerve stimulation at abdominal level (aVNS) for neurological conditions. We have shown that aVNS activates the brain region important for alleviating symptoms of a range of neurological conditions, including mental health disorders and epilepsy.”
– Dr Tomoko Hyakumura