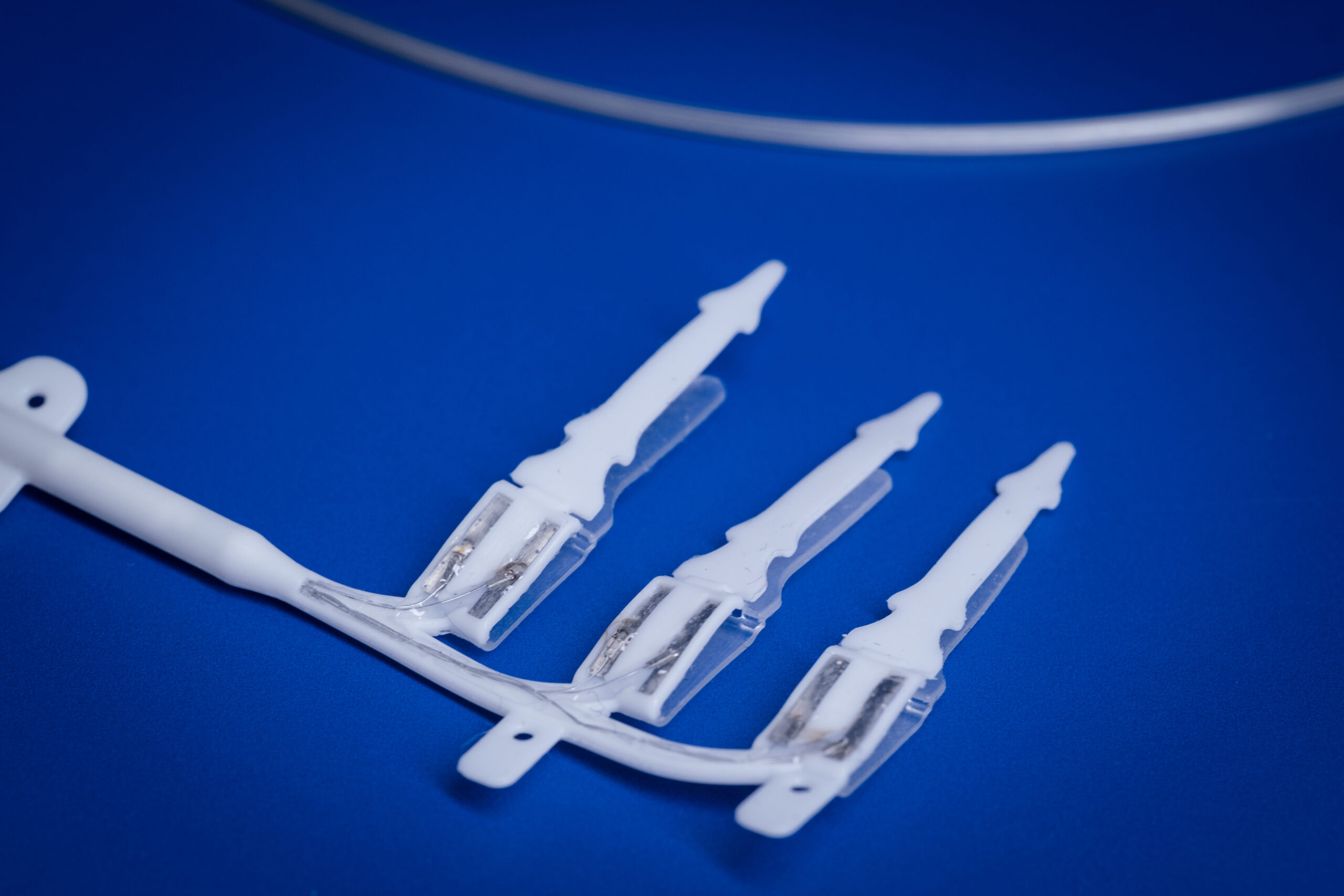
Latest News
Corporate giving in action: How RMS Australia turned expo engagement into impact
Corporate giving isn’t just about writing a cheque, it’s about creating meaningful engagement with customers, staff, and the broader community.
The Bionics Institute’s corporate partners are finding creative new ways to connect purpose with action, and RMS Australia recently delivered a standout example.
Fundraising with a twist
At the 2025 NoVacancy Hotel + Accommodation Industry Expo, RMS Australia turned their booth into more than a networking space and made it a hub for charitable giving.
Visitors to the RMS stand were invited to “check in for charity” by placing a button into one of several charity boxes, each representing a different cause. For every button dropped, RMS pledged a donation. It was a simple but powerful idea: empower attendees to direct corporate giving and make it interactive.
“Together, we raised $5,000 to support Bionics Institute, Foodbank Australia, Victorian Animal Aid Trust, and Orange Sky Australia, helping change lives (and wagging tails).” – RMS Australia via LinkedIn
This approach gave attendees a voice in the donation process, generated buzz around their booth, and showcased RMS’s commitment to making a real-world difference. From pioneering medical research to food relief, animal welfare, and mobile hygiene services.
How your business can get involved
Your organisation can make a difference too. Whether it’s:
- Hosting an interactive fundraiser like RMS
- Launching a staff giving program
- Sponsoring a specific research campaign
- Setting up workplace giving
The Bionics Institute makes it easy to align your company’s values with cutting-edge medical innovation.
Learn more or explore partnership options here:
bionicsinstitute.org/get-involved/corporate-giving-2025
You might be interested in…
Latest News
Bionics Institute CEO Robert Klupacs named finalist in 2025 InnovationAus Leadership Awards
We are proud to share that Robert Klupacs, Chief Executive Officer of the Bionics Institute, has been selected as a finalist in the 2025 InnovationAus Innovation Leadership Awards.
These awards recognise leadership excellence in Australia’s innovation ecosystem — celebrating people who can bridge vision and execution, and who inspire teams across disciplines to turn ideas into impact.
Distinguished peer finalists
Robert is one of three finalists in the Innovation Leadership Award category. The other two are:
- Professor Andrew Dzurak (founder & CEO, Diraq) InnovationAus.com
- David Lewis (founder & Managing Director, Mentor List Group) InnovationAus.com
According to InnovationAus publisher Corrie McLeod,
“Innovation is a team game, but there is always an outstanding leader at the helm, driving the project and ensuring its success. Congratulations to each of our finalists in the Innovation Leadership Award category.” InnovationAus.com
Robert’s nomination recognises his unique model for accelerating Australian innovation: a blended approach combining philanthropy, impact investment, and commercial intellectual property licensing.
His nomination statement emphasises that his enduring contribution lies in reshaping thinking within the medical research sector: that commercialisation is not a sideline, but a central path to sustainability, societal benefit, and translational impact.
Dual recognition: Epiminder’s finalist status
Our pride is further amplified by the fact that Epiminder Pty Ltd, founded by Professor Mark Cook AO and developed through collaborative work at the Bionics Institute, is also a finalist in the Health Tech category, for its Minder device. This recognises the strength of our innovation pipeline and partnerships across academia, hospitals, and industry.
The winners of the 2025 InnovationAus Awards will be announced on 27 November.
We extend our heartfelt congratulations to Robert, the Epiminder team, and all fellow finalists. Their leadership and ingenuity are driving the future of medical technology in Australia, and we are honoured to be part of that journey.
You might be interested in…
Latest News
Listening first: How consumers are shaping our device research
At the Bionics Institute, we’re proud to be partnering with Crohn’s & Colitis Australia and Arthritis Australia to ensure the technology we develop is useful and addresses unmet needs by hearing about the lived experiences of people with the conditions.
These partnerships mark a major step forward, creating formal consumer collaborations where people living with Inflammatory Bowel Disease or rheumatoid arthritis provide direct input into what we build, how trials are designed, and what outcomes matter most.
With consumer involvement, we hope to:
- Design devices that are useful and address unmet needs
- Define trials that are as inclusive and accessible as possible
- Provide educational platforms with information about our technology
The science behind the collaboration
We’re developing abdominal vagus nerve stimulation devices aimed at reducing inflammation in Crohn’s disease and rheumatoid arthritis. These small implants stimulate the vagus nerve to activate the body’s own anti-inflammatory response.
For Crohn’s disease, one of our devices is already being used by a clinical trial participant following bowel resection surgery.
For rheumatoid arthritis, we’re designing a similar device to help reduce chronic joint inflammation and have just commenced an initial clinical trial.
These innovations are led by Professor James Fallon and Associate Professor Sophie Payne, in collaboration with researchers from Austin Health, the Florey Institute and St Vincent’s Hospital Melbourne.
A pivotal shift toward individual-focused innovation
Setting up formal partnerships with Crohn’s & Colitis Australia and Arthritis Australia is a milestone in embedding lived experience into our research programs. It ensures that our technologies are useful and effective for as many people as possible.
By listening first and designing with those with lived experience, we’re moving closer to smarter, more effective treatments.
You might be interested in…
You might be interested in…
Latest News
Hannah Crawford appointed Interim Chair of the Bionics Institute Board
We are delighted to announce the appointment of Hannah Crawford as Interim Chair of the Bionics Institute Board.
Hannah has been an integral part of our governance team for several years as a Non-Executive Director and Chair of the Finance & Risk Committee. Her leadership, financial acumen and strategic expertise have been instrumental in supporting the Institute’s growth and ensuring robust oversight across all areas of operation.
She brings to this new role a wealth of experience in corporate finance, mergers and acquisitions, and organisational governance. Hannah began her career in chartered accounting with Arthur Andersen and Ernst & Young, and later spent over a decade at Grant Samuel, advising both listed and unlisted companies on strategic financial decisions.
In addition to her work with the Bionics Institute, Hannah currently serves as Chair of Local Guardians, Non-Executive Director of Confoil Australia & New Zealand, and a member of the Finance and Audit Committee of Melbourne Grammar School. She has also held directorships with Alfred Health, Australian Red Cross Lifeblood, and Neurosciences Victoria.
Her appointment as Interim Chair comes at an exciting time for the Bionics Institute, as we continue to expand the impact of our research and innovation in hearing, vision, neurological and chronic health conditions.
I am incredibly privileged to step into the role of Interim Chair of the Bionics Institute. Since becoming involved with the Institute in 2022, I have been continually astounded by the extraordinary work of our research team. It is an honour to help lead an Institute dedicated to transforming the lives of people living with challenging health conditions, and I am excited to contribute to shaping its future.
Hannah’s deep knowledge of our organisation and her passion for health and medical research make her ideally positioned to guide the Institute through this transition period. The Board is confident that her leadership will help steer the Institute through our next phase of development and discovery.
We thank Hannah for stepping into this vital leadership role and look forward to working closely with her as we continue delivering on our mission to improve lives through medical bionics.
You might be interested in…
Support Us
Invest in groundbreaking research to relieve chronic pain.
Donate nowWilliam’s Story
William hangs on to a vision of a future life…
One where he is free of pain from a ski accident two years ago… which left him with a paralysed arm and a constant agonising burning sensation.
One where he can throw himself into his work instead of often leaving when the pain gets too much for him to bear.
William hurt his arm in a skiing accident that led to a traumatic brachial plexus injury. Despite surgeries, medication, and attempts at alternative therapies, nothing has worked to relieve it.
“It’s a cruel thing, I’m grieving the arm… the pain is number one because it’s day in, day out… it’s hard to look down the track and stay positive”
William shouldn’t have to live with this pain forever. Especially when a pain-free life may be possible with groundbreaking new research.


New hybrid nerve stimulation to relieve chronic pain
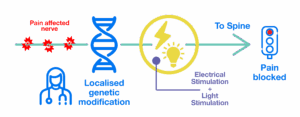
Specific cells are genetically modified to respond to light. This means we can target these cells (and only them!) with light. Our researchers are combining light and electrical current for highly targeted control of nerve activity that can be turned up to a higher level. This could mean more pain relief… and the potential of no negative side effects!
Help fund pioneering research
Chronic pain affects a staggering 1 in 4 people worldwide. Opioids, commonly prescribed for pain conditions, give inconsistent and often inadequate relief of symptoms, and can lead to serious side-effects, addiction, and overdose.
Caring researchers like Professor Rachael Richardson are pioneering a life-changing therapy. It’s called hybrid nerve stimulation – a world-first combination of light therapy and electrical stimulation.
This technique uses genetic modification to target pain signals without disrupting the surrounding nerves. It’s unlike current devices, which can activate the wrong nerves and cause spasms, shocks, or fail to work at all.
Your kind and thoughtful matched gift today will help move this research forward, bringing hope to millions suffering in chronic pain.


Watch our 2-min pitch about this research
Want to support research like this?
Want to support the future of research like this?
The progress of life-changing treatments are only made possible by donations from our supporters. Your support today could give those with chronic pain hope of a pain-free future.
Find out how you can support research innovation here.


Latest News
Celebrating Innovation, Leadership, and Legacy at the Bionics Institute
The Bionics Institute hosted a special Celebration Event in Melbourne’s CBD, bringing together key supporters, stakeholders, and esteemed guests, including Lord Mayor Nicholas Reece, to mark a defining moment in the Institute’s journey.
This significant occasion honoured the remarkable leadership of Mr John Stanhope AM, who is retiring after an extraordinary 10 years as Chair of the Board.
During his engaging speech, The Lord Mayor highlighted the central role of medical research in powering Melbourne’s economy and recognised John Stanhope’s outstanding contribution to the City of Melbourne through his leadership across major institutions and commended his influence on the Bionics Institute’s growth and success.
John Stanhope, during his tenure, guided the Institute through a period of transformative growth, establishing it as a global leader in bionic technologies and medical research.
Under John’s strategic leadership, the Bionics Institute has made groundbreaking advancements in treating hearing loss, Alzheimer’s, Parkinson’s, epilepsy, arthritis, and chronic pain. His vision helped drive the creation of four spin-out companies, including Epiminder, which received FDA approval for the Minder device developed at the Bionics Institute in April 2025.
John’s wealth of experience, including his roles as Group Managing Director of Telstra, Executive Director of the Telstra Board, Chairman of Australia Post, and current Chancellor of Deakin University, has been instrumental in shaping the Institute’s direction and amplifying its impact.
The event also served as a heartfelt thank-you to the Institute’s loyal donors and supporters, whose generosity fuels ongoing innovation and research. Their contributions continue to power the development of life-changing treatments and ensure a lasting legacy of hope for future generations.
As we celebrated past achievements and looked to the future, the event stood as a testament to the power of visionary leadership, scientific excellence, and community support in transforming lives through bionic medicine.
Read more about our research here: https://www.bionicsinstitute.org/our-research/
You might be interested in…
Latest News
Cybec Foundation Scholarship boosts chronic pain research
Bionics Institute PhD candidate Ethan Duff has been awarded a Cybec Foundation scholarship to progress his studies in the development of a device that could provide a new type of treatment for chronic pain.
“I am incredibly grateful to the Cybec Foundation for this generous scholarship,” Ethan said.
“This support will enable me to continue pursuing our promising investigations into pain signal transmission.”
Ethan is supervised by Professor Rachael Richardson, who leads the team in investigating the use of optogenetic techniques to selectively activate or suppress nerve activity.
Optogenetics is a technique that uses light to control the activity of nerve cells and can be combined with electricity to stimulate nerves in the development of new therapies for conditions such as hearing loss and chronic pain.
Ethan’s PhD studies involve using light-sensitive proteins to alter the transmission of pain signals before they reach the central nervous system, paving the way for a novel therapy for chronic pain that is more targeted than existing therapies.
“Chronic pain affects 1 in 4 people worldwide, and current medications are often inadequate,” he said.
“Developing a therapy that reduces the reliance on pain medications could have a huge impact on individuals living with chronic pain.”
To find out more about this research, go to: https://www.bionicsinstitute.org/our-research/autoimmune-and-chronic-disease/a-drug-free-approach-to-relieve-chronic-pain/
You might be interested in…
Latest News
Hybrid light and electricity therapy to combat chronic pain
We’ve all experienced pain. But dealing with chronic pain day in and day out for years on end affects every aspect of your life…your work, your mood, your sleep.
Chronic pain affects a staggering 1 in 4 people worldwide, but there is still no reliable treatment. Opioids, commonly prescribed for pain conditions, give inconsistent and often inadequate relief of symptoms, and can lead to serious side-effects, addiction, and overdose.
Nerve stimulators are available as an alternative to medication. Placed on the spinal cord or peripheral nerves, they use electricity to mask pain signals. While they can be effective, for many people, they fail to provide long-term benefits.
One reason for this is that it is extremely difficult to give enough stimulation using electricity to relieve pain without causing unwanted activation of other nerve fibres.
Bionics Institute researcher Professor Rachael Richardson and her team are investigating a potential treatment that combines the benefits of electrical stimulation with a novel, highly precise stimulus based on light that can be applied directly to the affected nerve. The combination of electrical and optical stimuli is called hybrid stimulation.
Our aim is to develop a hybrid stimulation device that suppresses pain with greater precision than pain medications and allows greater masking of pain than electrical-only nerve stimulators, transforming the lives of people with chronic pain. Prof Rachael Richardson
In their natural state, nerves cannot be stimulated by visible light, but Prof Richardson is a world leader in an emerging technology called optogenetics that uses a genetic modification in specific nerve fibres to make them sensitive to light.
The benefit of stimulating the nerve with light is that only the modified nerve fibres are activated while the other nerve fibres are completely unaffected, making the potential hybrid stimulation treatment much more precise.
In the context of pain, the highly precise neural signals generated by this potential hybrid stimulation therapy are processed in the spine, effectively giving pain signals a red stop light, while all the other neural activity have a green light, ensuring normal movement and sensation.

Prof Richardson’s team have had some excellent results from early research into this method and are now progressing this research to the next stage, which includes a plan to:
- Measure the effectiveness of hybrid stimulation on varying levels of chronic pain
- Develop clinically relevant tools to modify the affected nerves locally so they respond to light
- Engineer a nerve stimulator that can apply light directly to the nerve
- Perform safety studies with the aim of progressing the therapy to clinical trials.
Latest News
A Word From Our CEO – Spring 2025
Welcome to the Spring 2025 edition of The Current.
So much has happened since our last update, it’s been difficult to fit it all into one newsletter! Clinical trials with our devices in infants with hearing impairment, people with tinnitus, Alzheimer’s, Crohn’s and Parkinson’s disease continue to progress well, and we have reached major milestones in our early-stage and established research programs. You can find out more in the next few pages, where our lead researchers summarise their recent achievements.
In March, we launched the Vagus Nerve Stimulation Centre of Excellence at our Preview Event, officially opened by the Victorian Minister for Health, The Hon. Mary-Anne Thomas. This exciting initiative will enable our researchers to investigate the viability of a unique method of vagus nerve stimulation as a treatment for a wide range of conditions including gastrointestinal disorders, neurological disorders, mental health conditions, and even cancer.
In April, the Institute was delighted to receive the news that Epiminder’s Minder® device for epilepsy, pioneered by world-renowned neurologist Professor Mark Cook and Bionics Institute engineers, had gained FDA approval. This is an incredible testament to the commitment of a dedicated team of people, and we are all extremely proud that the
technology was originally developed here at the Bionics Institute.
In this edition, I’m very proud to describe a PhD Scholarship set up in partnership with my siblings to support a truly amazing young researcher named in honour of our parents. It really is a wonderful way to support the career of a promising researcher, and I hope that you may be willing to consider similar types of support.
If you would like to find out more about supporting the Bionics Institute, I encourage you get in touch and arrange to come for a tour – it’s fascinating!
As always thank you for your invaluable support, and I look forward to seeing you in our labs or at an event very soon.
Best wishes,
Robert Klupacs
Latest News
Research Update July 2025
We couldn’t do what we do without your generous support. Here’s a roundup of the progress we have made with your help.
Alzheimer’s disease
“Nearly 30 people have been enrolled into our clinical trial investigating the use of brain stimulation as a potential treatment for Alzheimer’s disease out of a total of 132, and we are continuing to develop the technology with the aim of delivering highly personalised therapy.”
– Professor Kate Hoy
Balance disorders
“My team in the NeuroMovement Laboratory is working closely with Bionics Institute engineers to develop wearable sensors that collect real-time data on movement and coordination, allowing for a more accurate assessment of a patient’s balance condition. It has the potential to detect early signs heightened falls risk, even before they become noticeable to the patient or healthcare provider.”
– Associate Professor David Szmulewicz
Chronic pain
“The Bionics Institute has been researching the use of a combination of light and electricity to improve cochlear implants. We recently started applying this technique to treat chronic pain, and early results are looking promising.”
– Professor Rachael Richardson
Crohn’s disease
“The first patient in the clinical trial of our vagus nerve device to prevent inflammation in Crohn’s disease has completed his 18 months in the trial and continues to feel fit and well. With the support of the Helmsley Charitable Trust we are continuing research into an innovative way to refine the device to provide adaptive stimulation.”
– Professor James Fallon, Chief Technology Officer
Rheumatoid arthritis
“In June this year, we launched our clinical trial to assess safety of vagus nerve stimulation and its potential benefits in reducing swelling and pain caused by rheumatoid arthritis. The Bionics Institute is the sponsor, and the trial is run in collaboration with our clinical team from St Vincent’s Hospital and the Austin Hospital.”
– Associate Professor Sophie Payne
Tinnitus
“We are collaborating with several clinicians to use our tinnitus diagnosis technology to monitor potential tinnitus treatments under investigation, with the aim of finding new ways to relieve symptoms.”
– Associate Professor Mehrnaz Shoushtarian
Infant hearing
“We are engaging with leading clinicians worldwide to refine our EarGenie test of infant hearing so that, once approved for clinical use, it will fast track intervention for infants with hearing loss to optimise their language development. I am excited to be one of the Founders of a newly created company, EarGenie Pty Ltd, which is currently completing its early fundraising activities, to be the vehicle to undertake ongoing engineering and clinical development and to ultimately market products to the audiology community throughout the world.”
– Professor Colette McKay
Hearing loss
“We are currently developing the process to manufacture a clinical grade drug delivery system for potential use in a clinical trial of our innovative therapy that aims to improve quality of life for people with hearing impairment.”
– Associate Professor Andrew Wise
Epilepsy
“We recently published a scientific journal paper on our early research into the potential use of vagus nerve stimulation at abdominal level (aVNS) for neurological conditions. We have shown that aVNS activates the brain region important for alleviating symptoms of a range of neurological conditions, including mental health disorders and epilepsy.”
– Dr Tomoko Hyakumura