A/Prof Andrew Wise, Associate Professor
A/Prof Wise graduated from Monash University (PhD) in 2002 where he undertook research in neuroscience.
He was recruited to the Bionic Ear Institute (2001-2003) as a post-doctoral research Fellow focusing on auditory neuroscience.
A/Prof Wise moved to Bristol UK where he took up post-doctoral position (2003-2006) before returning to Melbourne in 2006 to continue research at the Bionics Institute.
His research focuses on understanding hearing impairment and developing therapeutic technology to treat it.
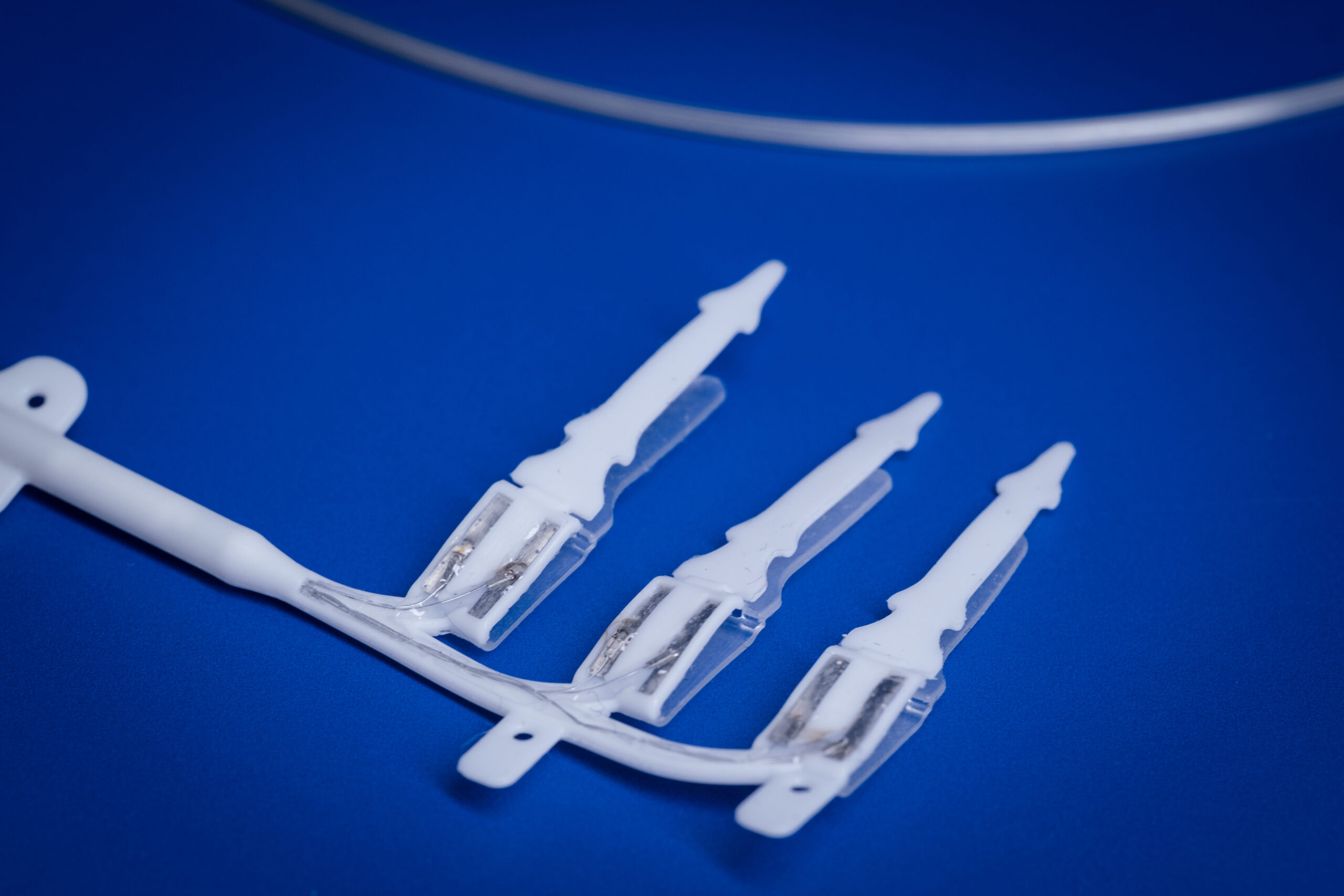
A/Prof Wise, his research team and collaborators have developed a new way to deliver therapeutic drugs to the inner ear to treat hearing impairment.
In particular, he is developing nanoparticle technology to deliver growth factors to protect the inner ear sensory cells from degeneration and promote repair following hearing loss.
His current research aims to progress this novel therapeutic approach to a first in human clinical trial that will enable the clinical translation of this exciting technology.
A/Prof Wise also has a research interest in cochlear implants and the development of new technologies to improve implant performance.
The delivery of therapeutic drugs could one day be used to improve cochlear implant performance by protecting residual sensory cells.
Furthermore, with the invention of optogenetic technology, it is now possible to modify auditory neurons so that they can be activated by light, in addition to electrical stimulation from a contemporary cochlear implant.
This new way of activating the auditory neurons could lead to significant improvements in a future bionic ear device, for instance, by improving the understanding of speech in noisy environments.
URL: https://www.bionicsinstitute.org/associate-professor-andrew-wise
ORCID: 0000-0001-9715-8784
Google Scholar: Andrew Wise
Recent publications
1. Gunewardene N, Lam P, Ma Y, Caruso F, Wagstaff S, Richardson RT, et al. Pharmacokinetics and biodistribution of supraparticle-delivered neurotrophin 3 in the guinea pig cochlea. Journal of Controlled Release. 2022;342:295-307.
2. Shepherd RK, Carter P, Dalrymple A, Enke YL, Wise AK, Nguyen T, et al. Platinum dissolution and tissue response following long-term electrical stimulation at high charge densities. J Neural Eng. 2021.
3. Richardson RT, Thompson AC, Wise AK, Ajay EA, Gunewardene N, O’Leary SJ, et al. Viral-mediated transduction of auditory neurons with opsins for optical and hybrid activation. Sci Rep. 2021;11(1):11229.
4. Thompson AC, Wise AK, Hart WL, Needham K, Fallon JB, Gunewardene N, et al. Hybrid optogenetic and electrical stimulation for greater spatial resolution and temporal fidelity of cochlear activation. J Neural Eng. 2020;17(5):056046.
5. Richardson RT, Ibbotson MR, Thompson AC, Wise AK, Fallon JB. Optical stimulation of neural tissue. Healthc Technol Lett. 2020;7(3):58-65.
6. Lam P, Gunewardene N, Ma Y, Caruso F, Nguyen T, Flynn B, et al. A radiolabeled drug tracing method to study neurotrophin-3 retention and distribution in the cochlea after nano-based local delivery. MethodsX. 2020;7:101078.
7. Hart WL, Richardson RT, Kameneva T, Thompson AC, Wise AK, Fallon JB, et al. Combined optogenetic and electrical stimulation of auditory neurons increases effective stimulation frequency-an in vitro study. J Neural Eng. 2020;17(1):016069.
8. Shepherd RK, Carter PM, Enke YL, Wise AK, Fallon JB. Chronic intracochlear electrical stimulation at high charge densities results in platinum dissolution but not neural loss or functional changes in vivo. Journal of Neural Engineering. 2019;16(2):026009.
9. Richardson RT, Hu Q-Y, Shi F, Nguyen T, Fallon JB, Flynn BO, et al. Pharmacokinetics and tissue distribution of neurotrophin 3 after intracochlear delivery. Journal of Controlled Release. 2019;299:53-63.
10. Pinyon JL, von Jonquieres G, Crawford EN, Duxbury M, Al Abed A, Lovell NH, et al. Neurotrophin gene augmentation by electrotransfer to improve cochlear implant hearing outcomes. Hearing Research. 2019;380:137-49.
11. Ma Y, Wise AK, Shepherd RK, Richardson RT. New molecular therapies for the treatment of hearing loss. Pharmacology & Therapeutics. 2019.
12. Ma Y, Cortez-Jugo C, Li J, Lin Z, Richardson RT, Han Y, et al. Engineering Biocoatings to Prolong Drug Release from Supraparticles. Biomacromolecules. 2019;20(9):3425-34.
13. Hart WL, Kameneva T, Wise AK, Stoddart PR. Biological Considerations of Optical Interfaces for Neuromodulation. Advanced Optical Materials. 2019;7(19):1900385.
14. Palmer JC, Lord MS, Pinyon JL, Wise AK, Lovell NH, Carter PM, et al. Comparing perilymph proteomes across species. The Laryngoscope. 2018;128(1):E47-E52.
15. Ma Y, Björnmalm M, Wise AK, Cortez-Jugo C, Revalor E, Ju Y, et al. Gel-Mediated Electrospray Assembly of Silica Supraparticles for Sustained Drug Delivery. ACS Applied Materials & Interfaces. 2018;10(37):31019-31.
16. Wise AK, Pujol R, Landry TG, Fallon JB, Shepherd RK. Structural and Ultrastructural Changes to Type I Spiral Ganglion Neurons and Schwann Cells in the Deafened Guinea Pig Cochlea. JARO. 2017.
17. Shepherd RK, Wise AK, Enke YL, Carter P, Fallon JB. Evaluation of focused multipolar stimulation for cochlear implants: a preclinical safety study. Journal of Neural Engineering. 2017;JNE-101771.R1.
18. Richardson RT, Thompson AC, Wise AK, Needham K. Challenges for the application of optical stimulation in the cochlea for the study and treatment of hearing loss. Expert Opinion on Biological Therapy. 2017;17(2):213-23.
19. Nayagam BA, Wise AK, Shepherd RK. The Auditory System. Neuroprosthetics. 2nd ed: World Scientific; 2017. p. 167-91.
See more from Andrew Wise at PubMed Google Scholar